| Shapes of Molecules | |
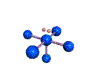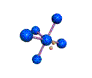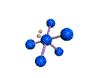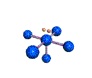 |
AX5E |
| Shape: | square pyramidal | |
| Steric Number: | 6 | |
| Lone Pair: | 1 | |
| Polar/NonPolar: | Polar | |
| Hybridization: | sp3d2 | |
| Example: | IF5 | |
| GIF File: | ax5e.gif (71K) |
| Return to Overview Page | |
| NOTES: This molecule is made up of 6 equally spaced sp3d2 hybrid orbitals arranged at 90o angles. The shape of the orbitals is octahedral. One orbital contains a lone pair of electrons so the remaining five atoms connected to the central atom gives the molecule a square pyramidal shape. |