| Shapes of Molecules | |
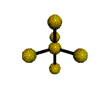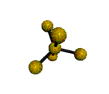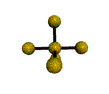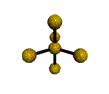 |
AX5 |
| Shape: | trigonal bipyramidal | |
| Steric Number: | 5 | |
| Lone Pairs: | 0 | |
| Polar/NonPolar: | NonPolar | |
| Hybridization: | sp3d | |
| Examples: | PCl5, SbF5 | |
| GIF File: | ax5.gif (96K) |
| Return to Overview Page | |
| NOTES: This molecule is made up of 5 sp3d hybrid orbitals. Three orbitals are arranged around the equator of the molecule with bond angles of 120o. Two orbitals are arranged along the vertical axis at 90o from the equatorial orbitals. The shape of the orbitals is trigonal bipyramidal. Since there is an atom at the end of each orbital, the shape of the molecule is also trigonal bipyramidal. |