| Shapes of Molecules | |
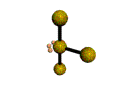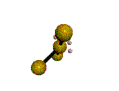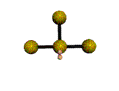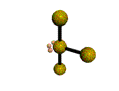 |
AX3E2 |
| Shape: | T-shaped | |
| Steric Number: | 5 | |
| Lone Pairs: | 2 | |
| Polar/NonPolar: | Polar | |
| Hybridization: | sp3d | |
| Example: | ClF3 | |
| GIF File: | ax3e2.gif (75K) |
| Return to Overview Page | |
| NOTES: This molecule is made up of 5 sp3d hybrid orbitals. Three orbitals are arranged around the equator of the molecule with bond angles of 120o. Two orbitals are arranged along the vertical axis at 90o from the equatorial orbitals. The shape of the orbitals is trigonal bipyramidal. Two of the equatorial orbitals contain lone pairs of electrons. The three atoms are arranged around the central atom to form a T-shaped molecule. |