| Shapes of Molecules | |
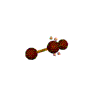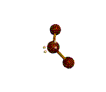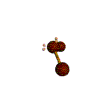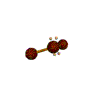 |
AX2E2 |
| Shape: | bent | |
| Steric Number: | 4 | |
| Lone Pair2: | 2 | |
| Polar/NonPolar: | Polar | |
| Hybridization: | sp3 | |
| Examples: | H2O, OF2 | |
| GIF File: | ax2e2.gif (52K) |
| Return to Overview Page | |
| NOTES: This molecule is made up of 4 equally spaced sp3 hybrid orbitals forming bond angles of approximately 109.5o. The shape of the orbitals is tetrahedral. Two of the orbitals contain lone pairs of electrons. The two atoms connected to the central atom form a molecule with a bent shape. |